Looking to help prevent costly recalls or audit failures? A well-designed and implemented Environmental Monitoring Program (EMP) is your frontline defense against contamination from dangerous pathogens such as Salmonella, Listeria monocytogenes, E. coli, and other pathogens.
Guided by regulations like the Food Safety Modernization Act (FSMA), an EMP is designed to proactively identify potential safety issues, enhance sanitary conditions, and ensure regulatory compliance, thereby safeguarding consumers and protecting brands.
This guide delves into the regulatory influences on your EMP, details the design and implementation process, and explores tools for automation, reinforcing your EMP as a crucial component of a comprehensive food safety management system.
Let’s get started.

What is an Environmental Monitoring Program?
An Environmental Monitoring Program (EMPs) is integral in ensuring product safety and regulatory compliance for food manufacturers.
They involve the systematic sampling and testing of the production environment for potential sources of contamination, such as pathogens, allergens, and other contaminants. Essentially, an EMP is a scientific method of validating the effectiveness of food safety systems.
What is the Purpose of an EMP?
The primary objective of EMPs is to prevent outbreaks of foodborne illnesses, which account for 48 million illnesses and 3,000 deaths annually, according to the CDC.
Environmental Monitoring Programs for food safety also facilitate compliance with regulatory requirements set by agencies like the FDA and USDA, align with the standards of GFSI-benchmarked food safety certification platforms, and help maintain product quality and brand reputation.

What are the Benefits of an EMP?
Implementing an effective Environmental Monitoring Program in your food manufacturing facility can yield several advantages:
- Contaminant Identification: EMPs can identify potential contaminants, such as pathogens or spoilage organisms, in the production environment, helping you direct your sanitation efforts and ensure product safety.
- Sanitation Verification: They can validate the efficacy of your sanitation processes and training programs, making sure these crucial safety measures are working as intended.
- Root-Cause Analysis: An EMP can help identify root-cause issues, leading to improved process controls and cost reductions.
- Hygiene Data Collection: They provide valuable data about the overall hygiene of your production facility, offering insights to inform further improvements.
- Equipment Maintenance: EMPs can uncover issues with equipment that require maintenance, helping your team address minor problems before they escalate into major challenges.
EMP Regulatory Requirements & Recommendations for Food Product Companies
There are several industry regulations, guidance documents, and best practices to consider when designing an Environmental Monitoring Program for food safety. Let’s look at a few of the most important.
FDA Compliance
Food and beverage manufacturers need to comply with the Food Safety Modernization Act (FSMA) and current Good Manufacturing Practices (cGMPs), with a focus on risk-based preventive controls. An Environmental Monitoring Program (EMP) is a pivotal tool to validate these controls and ensure cleanliness in various processing stages.
USDA Guidelines
For ready-to-eat meat and poultry products, USDA-FSIS guidelines, including 9 CFR part 430, the “Listeria Rule,” recommend EMPs as a means to verify sanitation processes, crucial for controlling pathogens like Listeria monocytogenes.
Almond Board of California Recommendations
The Almond Board of California, addressing concerns of Salmonella in almonds, advocates for an “aggressive” EMP as an effective verification tool for Salmonella controls.
GFSI Requirements
A Global Food Safety Initiative (GFSI)-benchmarked food safety certification is a significant demonstration of commitment to food safety. GFSI standards, including the Safe Quality Food (SQF) and BRCGS Food Safety codes, mandate EMPs, emphasizing its significance in the food manufacturing industry.
BRCGS and SQF specifically require documented EMP sampling plans, corrective actions, and trending data, which are critical components of audit readiness.
How EMPs Support HARPC Compliance
A well-designed Environmental Monitoring Program is a key element of your facility’s HARPC (Hazard Analysis and Risk-Based Preventive Controls) plan. The data from EMPs helps identify contamination risks and provides documentation to validate your preventive controls. By demonstrating that you’re actively monitoring for pathogens and taking corrective actions, you build a strong case for compliance with FSMA requirements.
Seek & Destroy: Identifying Potential Hazards with Your EMP
Effective Environmental Monitoring Programs should be designed to monitor and control potential sources of contamination, such as pathogens and other microorganisms.
Monitoring Pathogens
Pathogens like Salmonella, Listeria spp., E.coli O157:H7, and Cronobacter spp. are crucial targets. These pathogens have a significant potential to cause foodborne illnesses and should be closely monitored, especially in ready-to-eat (RTE) food facilities. Identifying potential harborage sites allows targeted sanitation efforts, reducing contamination risks.
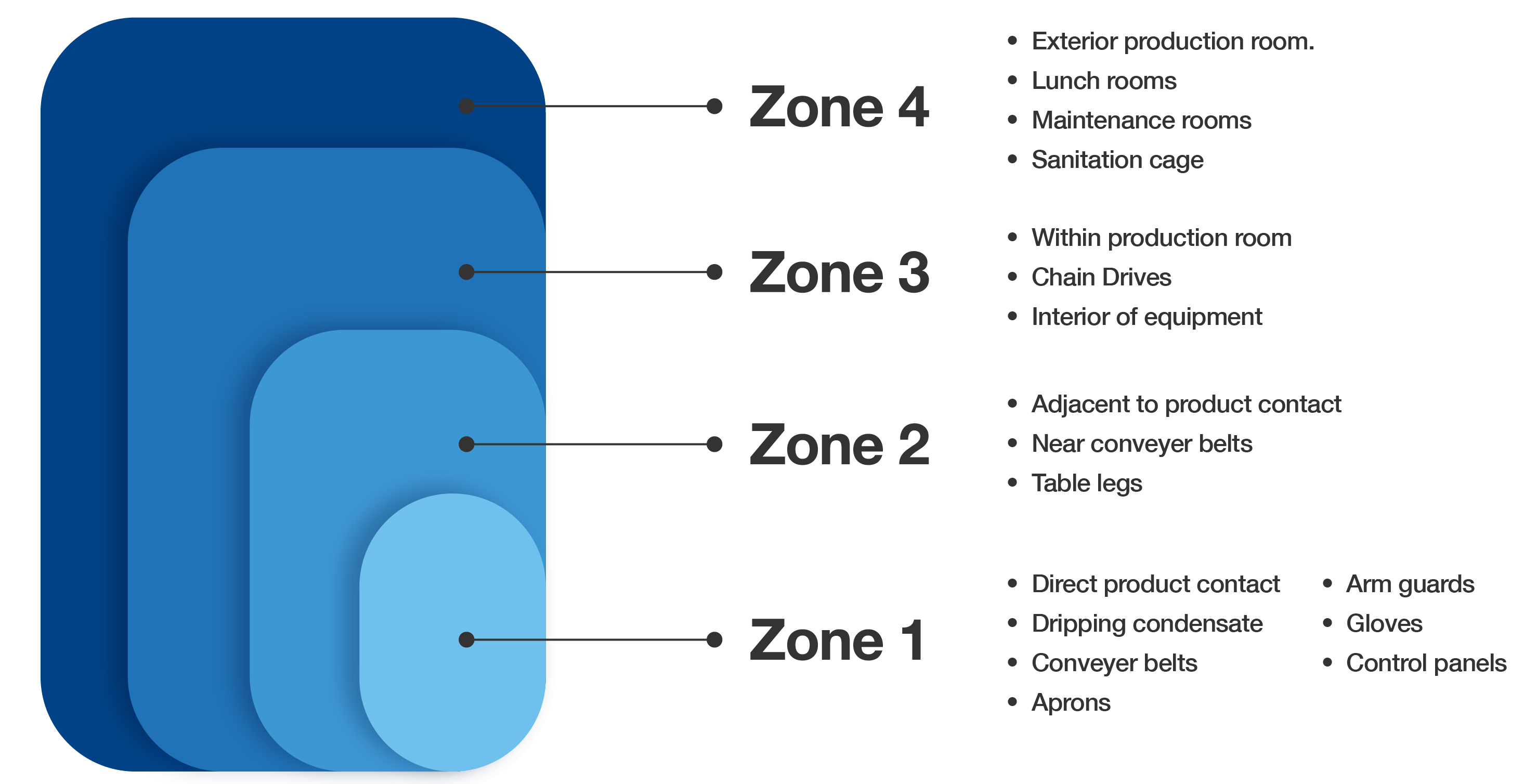
RTE facilities face greater scrutiny under USDA FSIS and FDA regulations due to their higher risk profiles. EMPs in these environments must prioritize Listeria spp., use aggressive zone 1–4 testing, and follow FSIS guidance under 9 CFR part 430, the Listeria Rule.

Spoilage Organisms
Monitoring spoilage organisms like Yeasts, Molds, and Lactic acid bacteria can prevent food spoilage, which can damage your brand and lead to waste. Regular monitoring can help identify sources of contamination and refine sanitation processes.
Indicator Organisms
Tracking indicator organisms such as Coliforms, Enterobacteriaceae, and Staphylococcus provides information about the hygienic state of your production facility, helping verify sanitation effectiveness.
Allergens
As food allergen-related recalls continue to rise, robust allergen testing is essential, especially if your facility handles raw materials that contain allergens.
Both the Food Safety Modernization Act (FSMA) and Global Food Safety Initiative (GFSI) standards require documented allergen controls. To meet these expectations, your EMP should include testing for the FDA’s “Big 9” allergens:
- Milk
- Egg
- Peanut
- Soybeans
- Wheat
- Tree Nut
- Fish
- Crustacean Shellfish
- Sesame
A practical and reliable method for detecting allergens is ELISA (enzyme-linked immunosorbent assay). This testing method offers high sensitivity and specificity at a cost-effective price point, making it a preferred tool in routine allergen verification.
FSNS also offers PCR testing (polymerase chain reaction) for allergens, which is ideal for baked goods and extruded snacks where proteins may be denatured or degraded.
Incorporating allergen swabbing into your EMP alongside pathogen and hygiene monitoring helps verify that cleaning procedures are effective at removing allergen residues. Focus swabbing efforts on high-risk areas like shared equipment, hard-to-clean surfaces, and transition points between allergen and non-allergen production runs.
By validating your sanitation practices with targeted allergen testing, you reduce the risk of cross-contact, support accurate labeling, and protect your brand from costly recalls.
Designing an Effective Environmental Monitoring Program (EMP)
An EMP is a systematic, science-based approach informed by risk assessment, aimed at early detection of product contamination. To ensure effectiveness, the program should adhere to FDA regulations and guidelines, consider potential contamination, risk, and entry points. Critical to this is the development of facility maps and sampling plans based on risk assessment.
Assembling a Team
Construct a diverse team of experts from departments such as Quality, Facilities, Production, and Microbiology. These members should have the capability to identify potential risks within their respective areas.
Regulatory Guidelines
Gather relevant regulations, guidance, and statutes applicable to your products and facilities, such as the Food Safety Modernization Act and industry-specific controls for pathogens like Listeria and Salmonella.
Conducting Risk Assessment
Assess potential contaminants in your production process. Tools like FMEA, HACCP, or Ishikawa tables can be used to identify contamination points, such as cross-contamination areas, raw food processing areas, potential harborage sites, high-traffic areas, and difficult-to-clean zones.
Designating Hygienic Zones
Divide production areas into four hygienic zones, each representing a different level of risk. This zoning influences sampling frequency and testing methods. Mapping these zones aids in identifying contamination sources and allows for the strategic implementation of an EMP.
Create an Effective Sampling Schedule
The efficiency of your Environmental Monitoring Program for food safety depends on the precision of your sampling schedule.
Your schedule should consider risk of contamination, facility complexity, and budget. To maximize the chances of detecting contamination, you should sample all zones of your facility frequently and adjust according to your observations. Random and discretionary sampling are both necessary for accurate results.
EMPs: When to Sample and Why
| Pre-Operation | When: After cleaning & before sanitation Why: Verify cleaning efficacy |
| First Shift | When: 3-4 hours into production Why: Verify that GMPs are effective; verify absence of embedded bacteria in equipment not reached by cleaning and sanitation that are exposed by the movement of equipment during production. |
| Second Shift | When: Prior to clean-up Why: Verify GMPs are effective; verify equipment does not have embedded bacteria that are exposed by the movement of equipment during production. |
| Investigation | When: Before cleaning and sanitation to identify harborage sites and determine how they are spreading via vector sample collection. Why: Usually done in response to multiple positives in a given area and when you need to determine the origin of contamination. |
Appropriate Sampling Tools & Techniques
The accuracy of your test results depends on proper sample collection. Large surfaces require sponges, while hard-to-reach areas require cotton-tip swabs. The collection method should neutralize chemical disinfectants on the sampling surface to preserve bacteria for accurate microbiology test results.
We compiled some environmental sampling best practices into this handy article. Here is a quick rundown:
- Use the appropriate swabbing tool
- Use the right swabbing technique
- Ensure adequate coverage area
- Incorporate neutralizing agents
- Identify critical areas to swab
- Use proper handing during sampling
- Maintain clean-to-dirty order
Selecting the Right Testing Methods
The choice of assays to perform depends on the applicable regulations and guidance for your products. Testing for pathogens, indicator organisms, and spoilage organisms can provide insights about the cleanliness and safety of your facility and its products.
When choosing between rapid tests like ATP and culture-based methods like APC or TPC, consider the trade-off between speed and microbial specificity. Many processors use ATP pre-operationally and plate counts for post-production trending.
The following provides a brief description of the common assays used by food and beverage processors for Environmental Monitoring Program testing:
- ATP (adenosine triphosphate): Effective for quickly monitoring general sanitation before production. Detects residual organic material remaining on surfaces following cleaning. Results are obtained in a matter of seconds.
- Coliforms and Enterobacteriaceae: Indicators of overall cleanliness. Include a wide range of microorganisms, including some that are pathogenic, making them effective indicator tests. Coliforms are often used as an indicator of potential presence of pathogenic E. coli, while Enterobacteriaceae are often used as an indicator of potential presence of Salmonella.
- Total Plate Count (TPC) and Aerobic Plate Count (APC): Provide an indication of the total microbial population on the test surface (both bacteria and fungi). Serve as excellent indicators of overall hygiene and the effectiveness of sanitation practices.
- Yeast & Mold: Indicates the presence of fungal spoilage organisms. Often used with TPC and APC to verify overall effectiveness of sanitation processes.
- Listeria spp. & Listeria monocytogenes: The most common assays performed for ready-to-eat food and beverage EMPs. Processors of many RTE foods must monitor for Listeria spp. to meet regulatory requirements. Both approaches are used, but monitoring for Listeria spp. casts a wider net than Listeria monocytogenes testing and may allow a producer to avoid dispositioning product until repeat positives are obtained, whereas an food contact surface that tests positive for Listeria monocytogenes will normally require a producer to automatically disposition the product.
- Salmonella: Facilities that process products susceptible to Salmonella contamination, such as low water activity foods like almonds and tree nuts, dry cereals, and peanut butter, as well as poultry, eggs, etc., should make this assay part of their EMP.
- Cronobacter: Processors of infant formula and other dried milk products are advised to monitor for this pathogen due to the high risk of contamination.

Setting Limits & Corrective Actions
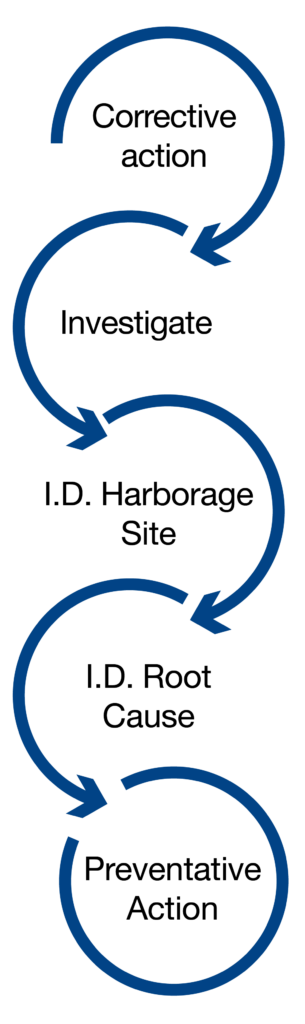
Limits and corrective actions are determined based on the risk posed by each test result. The detection of pathogens should trigger immediate corrective action. The response to contamination varies depending on the Zone of detection and could range from halting production to deep cleaning procedures.
If you get a positive result for pathogen testing in your Environmental Monitoring Program, here are the steps you should take:
- Determine the minimum number of vector samples to be collected after an initial positive.
- Pathogen positives require immediate, structured responses. Regulatory bodies expect detailed corrective actions to minimize contamination spread.
- Select vector sampling sites that represent areas and sites that could be the source of the initial positive findings.
- Follow-up samples should include the positive site and at least three surrounding sites, which could include food-contact surfaces (FCS) and non-FCS.
- Conduct vector sampling to determine the extent of contamination and establish potential root causes.
- Vector: 30 feet, 360°, high, and low.
- Use a “starburst” pattern when swabbing.
- Use deep cleaning procedures in follow-up to a positive test result.
- Resample the area around the original positive site daily to assess the effectiveness of the control measures.
- Obtaining three consecutive negative results as soon as possible from the day of presumptive positive detection is advised. This approach can help in promptly identifying whether the pathogen has spread beyond the initial detection site.
- Document the process and ensure proper disposition of any potentially contaminated product.
- Reconvene the risk assessment team to conduct a root cause investigation and uncover any unresolved issues that could lead to repeat positive findings.
Establishing a Baseline
Developing a baseline for your EMP involves determining the amount of microbial contamination in your facility. To establish a baseline, analyze swab results taken at different stages of the production process. Document all changes in real time to inform corrective actions and improvements to your EMP.
Trending EMP Data for Continuous Improvement
Once a baseline is established, use control charts or trend analyses to detect changes over time. GFSI schemes like SQF require trend analysis as proof of ongoing sanitation efficacy.
Using Predictive Analytics & Smart Monitoring
As digital EMP tools evolve, leading food safety teams are using EMP data and AI to predict where contamination might occur before it happens. By tracking swab trends over time and applying predictive analytics, you can anticipate risks and refine your sanitation schedule accordingly.
Check out some ways that AI is reshaping food safety in this complimentary on-demand webinar from our regulatory consulting arm, EAS Consulting Group, a Certified Group company.
Preparing for EMP-Related Audits
Beyond regular monitoring, your EMP should prepare you for audits, whether from the FDA, USDA, or a certification body performing a GFSI-benchmarked SQF, BRCGS Food Safety, FSSC 22000, or other audit. Key items to have on hand include:
- Sampling plans and zone maps
- Test results and trend analysis
- Corrective action records
- Training logs
- Pathogen testing protocols
A strong paper trail supports compliance and shows auditors you’re proactively managing food safety. We provide several tips to stay prepared for an unannounced GFSI-benchmarked audit here.
How to Implement an Effective Environmental Monitoring Program for Food Safety
Implementing an effective Environmental Monitoring Program necessitates a dynamic approach, continually evolving based on data analysis, modifications to production, and updates in regulations.
Ongoing EMP Team Training
Training should not be a one-off event but an ongoing commitment. It equips your EMP team with the necessary knowledge about your program, its purpose, and various technicalities.
Topics should cover…
- Suitable swabbing tools
- Neutralizer use
- Zones 1-4 swabbing strategy and technique, including vector swabbing
- Proper swab storage
- Documentation procedures
- Understanding of FSMA and GFSI environmental monitoring expectations
Food Safety Net Services (FSNS) offers training courses for your EMP team.
Importance of Comprehensive Documentation
Thorough documentation is a fundamental aspect of your EMP. Keep records of all facets, including…
- Sanitation Standard Operating Procedures (SSOPs)
- Corrective actions
- Target organisms
- Sampling methods
- Training records
These documents underpin your program’s effectiveness and ensure compliance with food certification audit platforms.
Periodic Review and Continual Improvement
Regular review and continuous improvement are crucial for your EMP’s effectiveness. The process should involve analyzing EMP results, staying updated with relevant regulatory changes, engaging employees in the review process, and creating improvement plans based on these inputs.
After implementing improvements, monitor their impact on your Environmental Monitoring Program. Analyze results to identify whether changes have reduced the incidence of contaminants, and use this data to further refine your EMP. The objective is consistent enhancement and adaptation to maintain optimal effectiveness.
Tools to Take Your Environmental Monitoring Program to New Heights
Your EMP will go a long way toward supporting your food safety programs. Here are a few Certified Group resources that can help you get more out of your EMP:
- FSNS and Certified Laboratories provide accurate microbiology and chemistry testing with competitive turnaround times.
- Your EMP will generate a lot of information that must be stored. Rather than using outdated binders and spreadsheets, our Environmental Monitoring and Mapping Application (emma®) provides real-time data visualization, customizable swabbing plans, and efficient remediation management.
- Improve EMP team performance with specialized training courses available throughout the year covering topics like Microbiology, Food Safety, and Sanitation.
- Design an efficient EMP with help from experts at EAS Consulting Group. Assistance includes determining sampling frequency, target organisms, and understanding relevant regulations.
- In case of persistent contamination, our contract research team can provide an on-site assessment and assist in identifying the source of contamination via microbial strain typing.
Your EMP will help your Quality Unit identify contamination and take corrective action before a small issue has a chance to become a big problem. It will also help you meet regulatory requirements and meet the requirements of food safety certification platforms, demonstrating your commitment to food safety.
Certified Group companies not only provide fast, accurate microbiology and chemistry testing in support of your EMP, we offer expertise and value-added solutions that help you manage and implement your EMP more effectively.
View all our EMP services and let us know how we can help you here.



