Reviewed By Kaci Foote, FSNS Certification & Audit Accreditation Manager
Our FSNS Certification & Audit team conducts hundreds of food safety certification audits each year, including BRCGS Food Safety audits, as well as other GFSI-benchmarked certification audits such as SQF and FSSC 22000.
Some sites have operated under GFSI certification programs for many years. Others are preparing for their very first BRCGS audit. Not surprisingly, the most common BRCGS audit findings differ between mature and new sites.
In this article, we outline the top five BRCGS audit findings observed at:
- Mature sites: Sites that have successfully completed at least one prior BRCGS certification audit and are in a recertification cycle.
- New sites: Sites in their initial BRCGS certification cycle.
All findings below are based on BRCGS Food Safety Standard, Issue 9, with applicable clause numbers noted for reference.
This guidance will help you prepare for your BRCGS audit, reduce non-conformances, and improve audit outcomes.
Top 5 BRCGS Audit Findings: Mature vs. New Sites
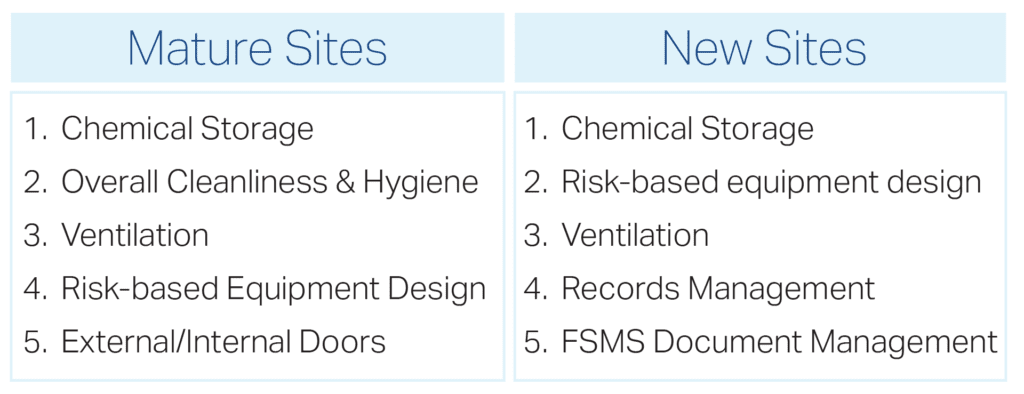
What are the Top 5 BRCGS Audit Findings for Mature Sites?
Mature sites typically have established programs, but long-term operations can allow small gaps to persist or gradually worsen over time. The following findings are most common in recertification audits.
#5) Internal and External Door Maintenance – 4.4.8
The clause states:
4.4.8 – Doors (both internal and external) shall be maintained in good condition. At a minimum:
- external doors and dock levellers shall be close fitting or adequately proofed;
- external doors to open product areas shall not be opened during production periods except in emergencies;
- where external doors to enclosed product areas are opened, suitable precautions shall be taken to prevent pest ingress.
Typical audit findings:
- Doors propped open during production.
- Damaged or missing door seals.
- Peeling paint or deteriorated surfaces.
- Gaps allowing pest entry.
Audit tip:
Before your BRCGS audit, conduct a facility walk-through focused specifically on all doors and dock interfaces. Verify physical condition, closure integrity, and pest-proofing.
#4) Risk-Based Equipment Design & Construction – 4.6.2
4.6.2 – The design and construction of equipment shall be based on risk, to prevent product contamination. For example, the use of the correct seals, impervious surfaces or smooth welds and joints, where they are exposed to product and could otherwise result in foreign body, microbiological or allergen contamination of the product. Equipment that is in direct contact with food shall be suitable for food contact and meet legal requirements where applicable.
Common issues observed:
- Rough or porous surfaces.
- Poor weld quality.
- Broken supports or structural damage.
- Materials not suitable for food contact.
These conditions can harbor microorganisms, allergens, and foreign material, increasing contamination risk.
Audit tip:
Evaluate equipment condition through a hygienic design lens. If contamination could reasonably occur due to surface condition or design, corrective action is required prior to your audit.
#3) Adequate Ventilation – 4.4.10
4.4.10 – Adequate ventilation and extraction shall be provided in product storage and processing environments to prevent condensation or excessive dust.
Common findings:
- Condensation on ceilings, walls, and overhead structures.
- Condensation above or near food contact surfaces.
- Insufficient airflow control.
Condensation represents a direct contamination risk when it can drip onto exposed product or contact surfaces.
Audit tip:
Document corrective actions for condensation control and verify effectiveness prior to the audit.
#2) Maintain Clean, Hygienic Equipment and Facilities – 4.11.1
The second more frequent BRCGS audit finding for mature sites relates to overall cleanliness and hygiene throughout the facility.
4.11.1 -The premises and equipment shall be maintained in a clean and hygienic condition.
Findings may include:
- Inadequate cleaning of production areas.
- Unsanitary utensils or tools.
- Poor employee hygiene practices.
Audit tip:
Perform a GMP and sanitation gap assessment using 21 CFR 117 and BRCGS expectations. Verify cleaning records and visual cleanliness on the production floor.
#1) Proper Storage and Handling of Non-Food Chemicals – 4.9.1.1
The number one BRCGS audit finding for mature sites involves non-food chemicals.
4.9.1.1 – Processes shall be in place to manage the use, storage and handling of non-food chemicals to prevent chemical contamination. These shall include, at a minimum:
- an approved list of chemicals for purchase;
- availability of material safety data sheets and specifications;
- confirmation of suitability for use in a food-processing environment;
- avoidance of strongly scented products;
- the labelling and/or identification of containers of chemicals at all times;
- a designated storage area (separate from chemicals used as raw materials in products) with access restricted to authorised personnel;
- use by trained personnel only;
- procedures to manage any spills;
- procedures for the safe, legal disposal or return of obsolete or out-of-date chemicals and empty chemical containers.
Key expectations include:
- Approved chemical list.
- SDS availability.
- Food-safe suitability confirmation.
- Proper labeling.
- Segregated, restricted storage.
- Trained personnel.
- Spill procedures.
- Disposal procedures.
Why this is the top finding:
Chemical-handling errors present a serious food safety and recall risk. Even experienced sites frequently overlook one or more of these control elements.
What are the Top 5 BRCGS Audit Findings for New Sites?
New sites often struggle with system implementation rather than physical facility issues. Documentation, records, and program control dominate early audit findings.
#5) Food Safety & Quality System Document Management – 3.2.1
3.2.1 – The company shall have a procedure to manage documents which form part of the food safety and quality system. This shall include:
- a list of all controlled documents indicating the latest version number;
- the method for the identification and authorisation of controlled documents;
- a record of the reason for any changes or amendments to documents;
- the system for the replacement of existing documents when these are updated.
- Where documents are stored in electronic form these shall also be:
- stored securely (e.g. with authorised access, control of amendments, or password protection);
- backed up to prevent loss.
Common gaps:
- No master document list.
- No version control.
- Missing change history.
- Inadequate electronic security or backups.
Audit tip:
Implement a structured document control procedure early. Once established, maintenance becomes significantly easier and reduces future audit findings.
#4) Records Management – 3.3.1
3.3.1 – Records shall be legible, maintained in good condition and retrievable. Any alterations to records shall be authorised and justification for the alteration shall be recorded. Where records are in electronic form these shall also be:
- stored securely (e.g. with authorised access, control of amendments, or password protection);
- suitably backed up to prevent loss.
Common findings:
- Illegible entries.
- Missing signatures or dates.
- Unauthorized changes.
- Poor electronic controls.
Audit tip:
If it is not documented, it did not happen. Ensure SOPs define how records are completed, reviewed, corrected, and stored.
#3) Adequate Ventilation – 4.4.10
4.4.10 – Adequate ventilation and extraction shall be provided in product storage and processing environments to prevent condensation or excessive dust.
This clause appears again for new sites. Condensation and airflow control remain universal challenges regardless of experience level.
#2) Risk-Based Equipment Design & Construction – 4.6.2
New sites frequently install equipment without fully evaluating hygienic design risk. The same contamination principles apply as noted in the mature site section.
#1) Proper Storage and Handling of Non-Food Chemicals – 4.9.1.1
Chemical control is the most consistent BRCGS audit finding across both new and mature sites.
Audit tip:
Review chemical SOPs, conduct staff training, and verify real-world compliance on the production floor prior to your audit.
Prepare for Your BRCGS Audit to Avoid Non-Conformances
Whether you are preparing for your first BRCGS audit or your 10th, proactive preparation is the most effective way to reduce BRCGS audit findings and achieve successful certification.
Key steps include:
- Conducting an internal gap assessment.
- Performing a formal BRCGS pre-audit.
- Reviewing historical non-conformances.
- Verifying SOP implementation on the floor.
Our team can perform a comprehensive pre-audit to identify gaps before your certification audit. As a 5-Star BRCGS Certification Body, we can also conduct your official BRCGS Food Safety audit when you are ready.
Contact us today to schedule your BRCGS audit or pre-audit and strengthen your audit readiness.



